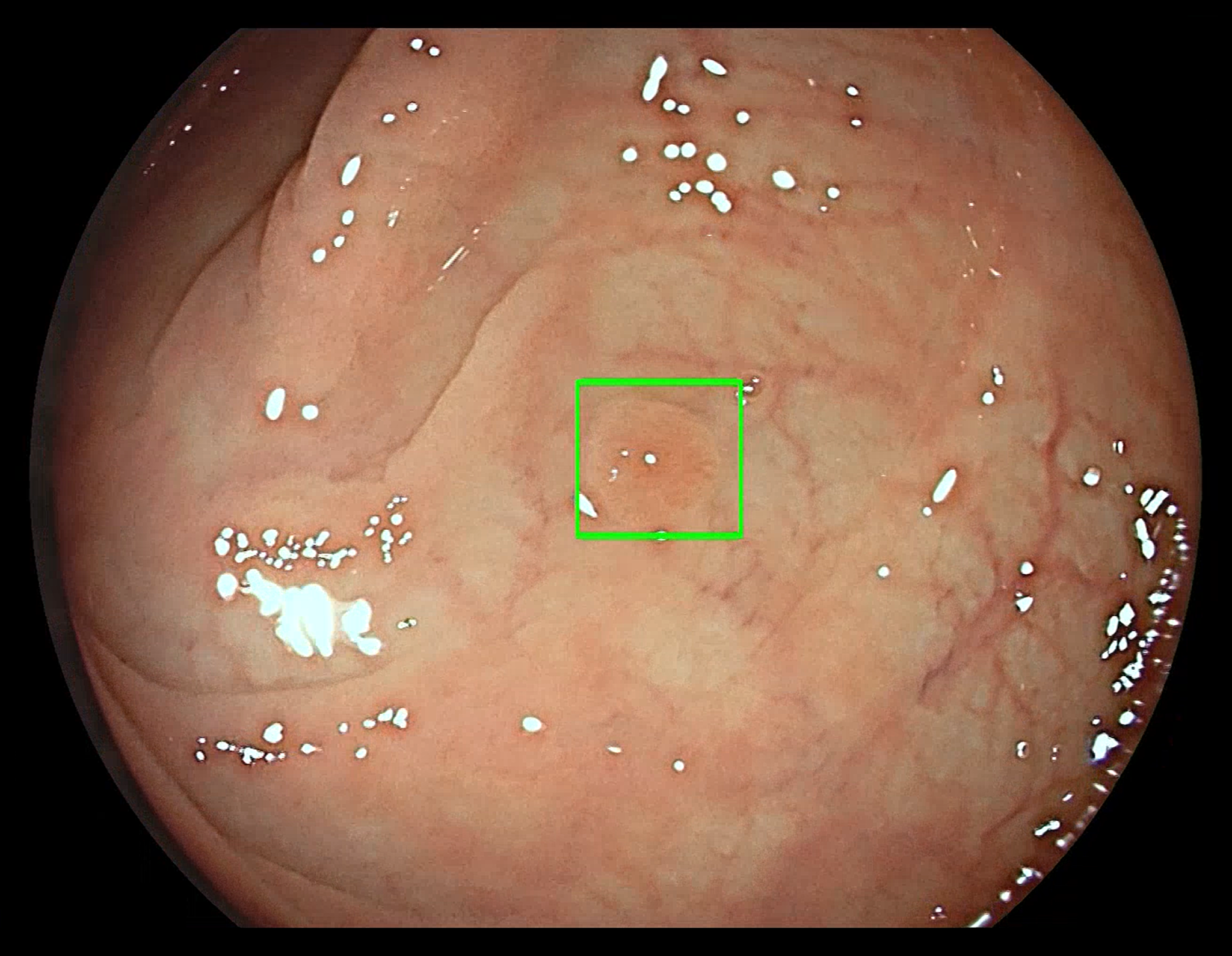
The COLO-DETECT study, which is looking at the ability of a new artificial intelligence device (GI Genius™) to help clinicians find polyps during a large bowel camera test (colonoscopy) – has taken a big step forward towards being ready to recruit its first participant.
The wellbeing of people participating in research is the paramount concern for those running any research study, and prior to getting up and running, a study must be reviewed by an NHS research ethics committee (REC) and the NHS Health Research Authority (HRA) to ensure that effective protection of participants' well-being has been made a top priority. Gaining approval from the REC and HRA for the study to begin is a vital step in the journey of the majority of research studies.
We are delighted that COLO-DETECT has recently been approved by both the REC and the HRA. It has also been adopted by the NIHR (National Institute for Health Research) as one of their 'portfolio' studies, meaning that it is entitled to additional support to help roll out and run the study in the participating hospitals. COLO-DETECT is also registered on 2 public databases (clinicaltrials.gov and ISRCTN) where anyone can read about the study, and also to improve accountability for conducting and reporting the research in the ways that are laid out in the study plan.
Now that all these things are in place, training of staff to deliver the study on the ground can begin and we expect COLO-DETECT to start recruiting participants soon – keep your eyes on the COLO-SPEED website and social media for more news!